lab chat: why the heck does drug development take so long?
Welcome to the latest round of Lab Chat, in which we try to demystify the often complex and mysterious field of biomedical research. Today, our conversant and well informed writer takes on commercial drug development -- specifically, why the heck does it take so long for prescription drugs to move from a scientist at a lab bench to the pharmacy at our local CVS?
From Bench to Bedside
The process of drug development is extremely long and complicated, not to mention enormously expensive. Estimates for moving a drug from “bench to bedside” can range in cost from $250 million to $1.5 billion and require roughly 10 to 12 years to complete. Not only is it time consuming and expensive, it’s extremely difficult. Only 1 out of every 1,000 drugs tested in the lab ever make it to the marketplace, and even those that advance to clinical trials have only a 1-in-5 success rate. This is due to the extremely rigorous regulations that the Food and Drug Administration (FDA) enforce to ensure that drugs are safe and effective.
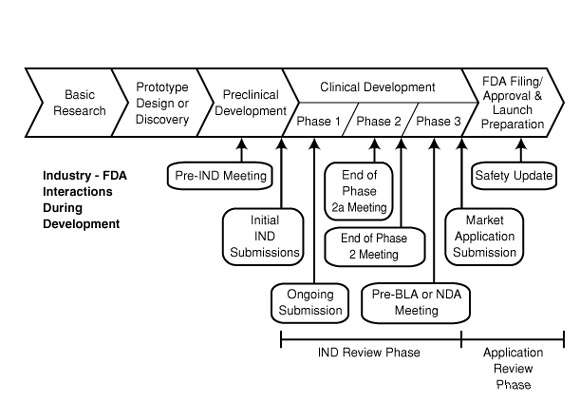
What follows are the general phases of drug development and the hurdles that must be crossed in each before moving forward.
(1) Pre-Clinical Development (3-5 year average)
Let’s say hypothetical scientist Dr. Pharma identifies a rare compound in sparkling Lake Erie that clears up acne in mice on contact. He spends the first two years following the discovery proving to the scientific community, via peer reviewed publications, that the compound clears up acne in mice. He identifies that it does so by increasing blood flow to the center of the pimple, which then gets attacked and cleared by the immune system.
Well, that’s it, right? Dr. Pharma is now the world’s newest billionaire and pubescent children everywhere will forever sing his praises. Wrong! Remarkably, what the doctor has accomplished so far actually is the easy part. The next three or so years of his life will be spent characterizing everything about the drug during this pre-clinical development phase.
One of the initial steps Dr. Pharma will have to complete is to characterize the “ADME” of his new drug. ADME stands for “absorption, distribution, metabolism and excretion.” The absorption of the drug refers to how it will reach the target tissue. Can it be orally administered and absorbed in the bloodstream or is it too unstable a compound and thus requires administration through IV or inhalation? The distribution of a drug refers to how it will reach the target tissue (in this case the center of Mt. Vesuvius). When a drug enters the bloodstream it doesn’t have a GPS and often will reside in multiple organs before finally arriving at its destination. Factors influencing the distribution of a drug include regional blood flow, pH stability of the compound, plasma protein binding of the drug, and a plethora of others. The metabolism of the drug refers to the body’s response to the drug and subsequent breakdown into smaller components called metabolites. The metabolism of a drug can affect its duration of efficiency, and a drug that’s broken down too quickly might never reach its target tissue. Additionally, drugs can have metabolites that cause complications that lower the safety of the drug. The excretion of the drug is exactly what it sounds like: How does the drug leave the body? Is it processed in the kidney and removed via urine, is it passed from the liver to the gut and excreted as feces, or is the main method of excretion through the lungs?
The ADME is just a small sampling of the studies that will need to be performed on a new drug. Dr. Pharma also must detail the toxicology of the drug, i.e. is it safe? At what dose does the drug stop being effective and start being toxic? The goal of these studies is to identify the “therapeutic index” of the drug, that is, the range of doses at which the drug is effective and still safe. Another factor to consider is whether or not the drug can be manufactured in large quantities at high purity. One thing I neglected to mention about Dr. Pharma’s compound is that he had to filter 5,000 gallons of Lake Erie water to isolate 0.005 mg of compound. Thus, to purify the thousands of grams necessary to synthesize suitable amounts for all those greasy teens, we’re going to have to drain all of Lake Erie and a portion of the Atlantic Ocean as well. Uh oh, we might have just hit a roadblock.
We could go on about the additional studies our scientist will have to complete during this phase (including a thorough characterization of pharmacodynamics and bioanalytical testing), but it's best we keep things moving.
(2) Submission of Investigational New Drug Application
Well, here we are five years into his discovery and Dr. Pharma finally has all the answers he needs to move this drug to the next phase of testing. He'll need to submit an application to the FDA called the investigational new drug application (IND) to seek permission to test his drug in humans. In this application the investigator needs to make it abundantly clear to the FDA that the drug should be safe for testing in humans by providing details on everything performed in the pre-clinical development stage, namely the pharmacology, toxicology and manufacturing information, not to mention a clinical protocol and signed agreements to adhere to all clinical testing regulations. The FDA then has 30 days to review the IND for safety and to ensure that the potential research subjects will not be subjected to drug-associated risks.
(3) Phase 1 Clinical Trial (1 year average)
Now that we’ve received FDA approval to begin drug testing in human patients it’s time for Phase 1 clinical trials. The main purpose of Phase 1 testing is to determine the safety profile of the drug in humans. Up to this point, all of Dr. Pharma's work has been done in animal models and thus the effect in humans still is an unknown. Working with physicians at a hospital, the clinical trial team will assess the safety and tolerability of the drug in a healthy patient population, and perform dose escalation studies to identify the safest dose that can be administered to patients. Phase 1 trials usually are small, with only 20 to 50 healthy patient volunteers, and the ultimate goal is ensuring patient safety by understanding the ADME of the drug in humans. While this trial might seem straightforward, only 60 to 70 percent of drugs that enter Phase 1 make it through to Phase 2. Much of this failure rate can be attributed to the drug behaving differently in the human system than the animal model used during pre-clinical phases.
(4) Phase 2 Clinical Trial (2 year average)
Now that we’ve established a safety profile of the drug in humans, it’s time to actually see if we have an effect in the desired patient population. Phase 1 was performed in healthy (in this case, acne-free) patients and thus we didn’t learn anything about its biological effectiveness. In Phase 2, a larger patient sampling (between 50 and 300) with the particular disease/condition will be treated with the drug and its effectiveness will be compared to a placebo treatment. A well-defined endpoint for clinical efficacy must be met in order to deem Phase 2 trials a success, and the ongoing safety studies from the Phase 1 trials must continue to show safety and tolerability. Phase 2 trials often are the point where a drug fails to show efficacy over the placebo control, with only roughly 30 to 40 percent of drugs advancing to Phase 3 trials. The failure rate often can again be attributed to a certain drug working wonders in mice but not in humans. However, if all does go as hoped and the drug proves effective in clearing the skin of patients, the clinical trial team again meets with the FDA to determine how to scale up the process and move forward to Phase 3.
(5) Phase 3 Clinical Trials (3 year average)
Well, we’ve made it to Phase 3 trials and that light at the end of the tunnel finally is coming into view. We’ve got just a few more hurdles to jump before Dr. Pharma's drug can hit the market, but this one is high. Phase 3 clinical trials escalate the patient population to between several hundred and 3,000 or so, and now the test is on to see if the new drug is more effective than the current standard of care. These studies are extremely expensive to conduct as they are performed at multiple institutions around the world and can last many years. In this case Dr. Pharma's drug might be directly competing against a previously FDA-approved acne treatment such as benzoyl peroxide. During these trials, not only is the efficacy of the drug compared but also factors like quality of life and side effects, plus the ongoing safety studies that began during Phase 1. The success rate of Phase 3 trials also is not very high, with approximately 40 percent of drugs failing to advance to the next stage. Part of that failure rate can be attributed to the new drug not being more effective than the current standard of care. However, if Dr. Pharma -- who is now a decade older and likely scarred from the process -- is able to make it through this stage, he can finally see the light at the end of the tunnel.
(6) Submission of New Drug Application (NDA)
If the new drug flew through Phase 3 and was shown to be more effective than the prior standard of care, Dr. Pharma will submit a new drug application (NDA). This is a document detailing every piece of information that the team has generated on the drug, beginning at the pre-clinical stage all the way up to completion of the Phase 3 trial. That’s about 12 years of work condensed into a document several thousand pages long.
The folks at the FDA will take about six to 10 months to review every word of the NDA to assess whether the drug has passed its strict requirements on safety, efficacy and manufacturing. Assuming all goes well with the application, Dr. Pharma finally gets to pop that bottle of Moet and celebrate, for he’s managed to push a drug from bench to bedside.
While this certainly is a lot of technical information to digest, one now gets a sense of just how difficult it is to translate something exciting folks are working on in a Cleveland lab into a standard of care treatment to be used in the clinic. While the process is convoluted and often frustrating, the safety standards are set in place to ensure that patients receive the utmost quality care without the risk of an untested product.
Amar Desai is a Post-Doctoral Research Associate in the Department of Hematology/Oncology at Case Western Reserve University. He works in the field of adult stem cells and regenerative medicine with the goal of developing novel therapeutics for hematological diseases in hopes of one day popping his own bottle of Moet. You can contact Amar here.