This year's Medical Innovation Summit will highlight neurosciences innovation
Thousands of researchers, investors, entrepreneurs and those who are simply curious will descend upon the Cleveland Convocation Center October 26-28 for Cleveland Clinic Innovations Medical Innovation Summit. This year’s theme, The Neurosciences: Memory. Mood. Movement, will cover all aspects of the human brain and how it functions and malfunctions.
“This is our marquee event,” says Thomas Graham, Cleveland Clinic’s chief innovation officer and this year’s emcee. “It’s in its 13th year and all the important stakeholders will be in town.”
Graham refers to the summit as the intersection where knowledge, different perspectives and resources come together with inventors, investors and medical industry representatives. “Really, when you bring ideas together and people have discourse, breakthroughs happen,” he says.
 Thomas Graham, MDThree CCI companies and research groups in the neurosciences have made significant strides in Alzheimer’s disease, concussion prevention and treatment, and drug administration to the human brain. This group of doctors and researchers represent just a small sample of what the Medical Innovation Summit has to offer this year.
Thomas Graham, MDThree CCI companies and research groups in the neurosciences have made significant strides in Alzheimer’s disease, concussion prevention and treatment, and drug administration to the human brain. This group of doctors and researchers represent just a small sample of what the Medical Innovation Summit has to offer this year.
“This is a great selection,” says Graham. “We bring forth important advances in technique and technologies.” These technologies are in the process of going to market. All three companies will present in some form at the summit.
A step toward treating Alzheimer’s
After 10 years of research into treatment for neuropathic pain – pain caused when damaged nerves in the body send false pain signals to the brain – Mohamed Naguib in the Cleveland Clinic’s anesthesiology department discovered an additional benefit to the treatment: It may also help slow the progression of Alzheimer’s disease and other neuro-inflammatory diseases.
Neuropathic pain often occurs in amputees with what is known as “phantom limb syndrome,” chemotherapy patients and diabetics with nerve damage. “Things like a light touch or change in temperature can become very painful,” explains Joseph Foss, director of clinical research in the Clinic’s Anesthesiology Institute. “The nerves are sending a signal to the glial cells in the brain, which release inflammatory agents that cause the nerve cells to become damaged.”
 Joseph Foss, MDThis process led Naguib and Foss to look at the effects of an abnormal protein in the brain, amyloid, which builds up in Alzheimer’s patients and causes brain inflammation.
Joseph Foss, MDThis process led Naguib and Foss to look at the effects of an abnormal protein in the brain, amyloid, which builds up in Alzheimer’s patients and causes brain inflammation.
Naguib and Foss think they have discovered a compound that will disrupt the inflammation associated with the buildup of the amyloid and thereby prevent or slow symptoms of Alzheimer’s, Parkinson’s, Multiple Sclerosis and other neurological diseases. Dubbed MDA7, the compound reduced neuro-inflammation and stopped the progression of such conditions in animal tests.
Foss and Naguib just last week finalized the formation of NeuroTherapia, a spinoff company out of Cleveland Clinic Innovations that will hopefully bring MDA7 to market. “It’s a situation that encompasses many diseases,” says Naguib, adding that patients with a family history could be treated before symptoms even arise. “The earlier you can reach any of these groups, hopefully we can increase the quality of life.”
 Mohamed Naguib, MDThe pair received a $250,000 grant from the Alzheimer’s Drug Discovery Foundation earlier this year, and then received another $600,000 from the organization’s fundraiser in April. CCI provided $1 million in funding for NeuroTherapia’s research, “which puts us about halfway to where we need to be,” says Foss. “We’re not only looking at potential grants, we’re looking into philanthropic sources and angel investors.”
Mohamed Naguib, MDThe pair received a $250,000 grant from the Alzheimer’s Drug Discovery Foundation earlier this year, and then received another $600,000 from the organization’s fundraiser in April. CCI provided $1 million in funding for NeuroTherapia’s research, “which puts us about halfway to where we need to be,” says Foss. “We’re not only looking at potential grants, we’re looking into philanthropic sources and angel investors.”
Foss and Naguib are hesitant to call MDA7 a cure for Alzheimer’s, but rather part of a cure. “It’s a little like saying there’s a cure for cancer,” explains Foss. “We don’t have a cure out there, we have a couple different approaches. I think we are still working to understand all of the components that go into developing Alzheimer’s disease. It’s a cascading effect. Our hope is we can significantly change the course of the disease.”
NeuroTherapia is currently manufacturing MDA7 for additional animal safety studies. “We are moving ahead with our work,” says Foss.
A better approach to concussions
The importance of recognizing concussions and determining the subsequent treatment has received a lot of attention in recent years. Mild traumatic brain injuries can affect nearly anyone, and the lasting effects can be debilitating.
Since 2011, a team at the Cleveland Clinic Neurological Institute has been researching the consequences of concussions. Led by Jay Alberts, director of the Clinic’s Concussion Center and a member of the biomedical engineering staff at the Neurological Institute, the team has developed a mobile app called Cleveland Clinic Concussion App (C3). The app collects data on balance, reaction time, memory and vision for athletes after a hit. It then compares that information to data previously collected on that player in a normal state. The app can be used to determine if that player can return to the game or has to receive medical treatment.
“It’s used in all the high schools and colleges where Cleveland Clinic sees [patients],” says Susan Linder, a member of Alberts’ research team. “It records how kids are when they’re healthy before the season begins, and then if they get a concussion they use it to compare. It’s an aid in allowing trainers and clinicians to determine treatment.”
The app helps with timing and course of treatment, says Linder. “It directs care from a research perspective,” says Linder. “The only focus in a clinical environment is to get student athletes to the right level of care in a timely manner.”
Aside from rest, there is not a lot known about what helps best in recovery from a concussion, says Linder, who says the typical recovery time is around a week to 10 days but some patients still experience symptoms for much longer periods. Impairments include headaches, memory problems and cognitive function.
“A concussion is a brain injury and patients are missing school time or work time,” explains Linder. “When you can’t do that for a month, that’s significant for them. Why don’t we take the same model and address those symptoms before they become chronic?”
CCI assisted with the commercialization of the C3 app.
Breaking the barrier to fighting brain tumors
There are a variety of approaches to treating cancerous tumors in the body. But when it comes to treating brain tumors, traditional methods do not usually work. The brain protects itself from toxins and other agents in the rest of the body and the Blood Brain Barrier (BBB) allows certain elements to get into the brain, while keeping others out – including chemotherapy and anti-cancer drugs.
 Michael Vogelbaum, MD“One of the biggest problems we face in delivery of products to the brain is most therapeutics are kept out of the brain by the Blood Brain Barrier,” explains Michael Vogelbaum, associate director of the Clinic’s Brain Tumor and Neuro-Oncology Center and a member of the Neurological Surgery staff.
Michael Vogelbaum, MD“One of the biggest problems we face in delivery of products to the brain is most therapeutics are kept out of the brain by the Blood Brain Barrier,” explains Michael Vogelbaum, associate director of the Clinic’s Brain Tumor and Neuro-Oncology Center and a member of the Neurological Surgery staff.
For decades, doctors have tried to get tumor-shrinking drugs into the brain, but most of those methods have failed. Vogelbaum notes that direct-to-brain methods, such putting the drugs into the spinal fluid, have seen limited success. Catheters designed to directly administer drugs to the brain had seal problems – if the catheter doesn’t have a good seal, the drugs don’t go where they need to go.
“Delivery to the brain is not as simple as it sounds because the brain resists the infusion,” he says. “This has been a huge unmet need in neuro-oncology.”
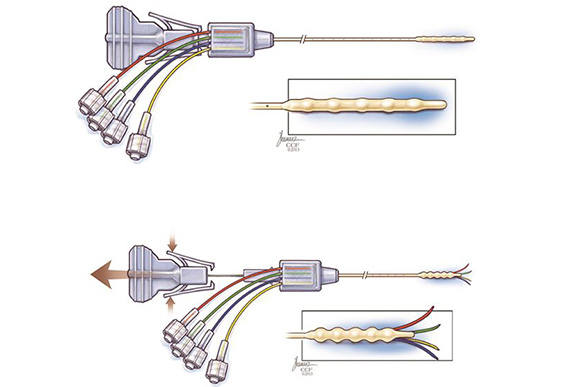
Vogelbaum was brought on by CCI in 2009 to lead a team of scientists, biomedical engineers, a patent attorney and a business development officer in developing the “cat’s paw” Cleveland Multiport Catheter (CMC), in which two small catheters come off a larger, central catheter.
The CMC delivers drugs directly to the brain with greater success than previous catheter models and other traditional methods. The CMC uses convection enhanced delivery (CED) with implanted catheters and pumps.
After testing the new catheter, Infuseon Therapeutics was created by CCI to commercialize the product and the company secured FDA Investigational New Drug (IND) approval for use of the MRI contrast agent gadolinium and the chemo drug topotecan with the catheter.
Three patients have been treated through clinical trials and Vogelbaum is about to open a new study to explore different delivery rates. “What we’re focusing on is a lot of technical questions in terms of using this device,” he says. “We‘re evaluating the efficacy of various drug combinations.”
The human trials so far have proven promising. “We’re taking it one step at a time,” says Vogelbaum. “Ultimately, what’s important is the thought that we can realize real clinical benefits.”
Vogelbaum says there has already been significant interest in the CMC. In addition to treating brain tumors, Vogelbaum says there are potentially other diseases that could be treated using the catheter, including epilepsy and strokes.